
Celiac.com 10/14/2013 - A team of researchers recently set out to assesses the safety and efficacy of Aspergillus niger prolyl endoprotease (AN–PEP) to mitigate the effects of gluten in celiac patients.
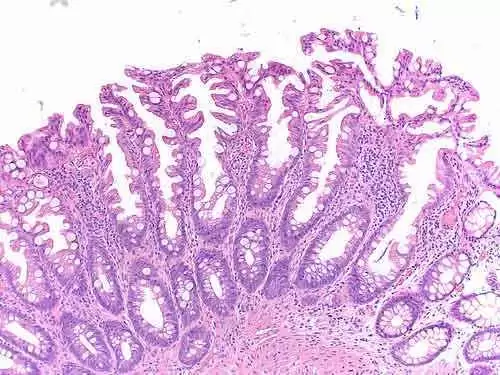
 For their study, the researchers included celiac patients with positive serology and subtotal or total villous atrophy on duodenal biopsies, who follow a strict gluten-free diet (GFD) resulting in normalised antibodies and mucosal healing classified as Marsh 0 or I were included.
For their study, the researchers included celiac patients with positive serology and subtotal or total villous atrophy on duodenal biopsies, who follow a strict gluten-free diet (GFD) resulting in normalised antibodies and mucosal healing classified as Marsh 0 or I were included.
Celiac.com Sponsor (A12):
Prior to this randomized double-blind placebo-controlled pilot study, the team measured complaints, quality-of-life, serum antibodies, immunophenotyping of T-cells and duodenal mucosa immunohistology.
They then had patients consume approximately 7 grams of gluten per day as toast, along with AN-PEP for a two week safety phase. The team put subjects through a two week washout phase where they followed their usual gluten-free diets. The team then randomly assigned 14 patients to receive gluten, with either AN-PEP or placebo, for a two week efficacy phase.
They also collected patient questionnaires on serum and quality of life during and after the safety, washout and efficacy phase. They conducted duodenal biopsies after both the safety phase and the efficacy phase. Change in histological evaluation according to the modified Marsh classification served as the primary endpoint.
In all, 16 adults participated in the study. No serious adverse events occurred during the trial and no patients withdrew during the trial. The average score for the gastrointestinal subcategory of the celiac disease quality (CDQ) was relatively high throughout the study, indicating that AN-PEP was well tolerated.
In the efficacy phase, the team saw no significant deterioration in the CDQ scores of patients consuming gluten with placebo or gluten with AN-PEP, nor did they observe any other differences between the groups. During the efficacy phase, neither the placebo nor the AN-PEP group showed significant antibody titers. IgA-EM concentrations remained negative in both groups.
The team excluded two patients from entering the efficacy phase because their mucosa showed an increase of two Marsh steps after the safety phase, yet with undetectable serum antibodies. A total of 14 patients were considered histologically stable on gluten with AN-PEP.
Also, after the efficacy phase, the team saw no significant deterioration in immunohistological and flow cytometric evaluation in the group consuming placebo compared to the group receiving AN-PEP.
Furthermore, in four out of seven patients on placebo, IgA-tTG deposit staining increased after two weeks of gluten intake compared to baseline.
In the seven patients receiving AN-PEP, one patient showed increased and one showed decreased IgA-tTG deposits.
AN–PEP appears to be well tolerated. However, the primary endpoint was not met due to lack of clinical deterioration upon placebo, impeding an effect of AN–PEP.
The research team included Greetje J Tack, Jolanda MW van de Water, Chris J Mulder of the Department of Gastroenterology and Hepatology, VU University Medical Centre in Amsterdam, The Netherlands; Engelina MC Kooy-Winkelaar, Jeroen van Bergen, and Frits Koning of the Department of Immunohematology and Blood Transfusion, Leiden University Medical Centre in Leiden, The Netherlands; Petra Bonnet, B Mary E von Blomberg, and Marco WJ Schreurs from the Department of Pathology, VU University Medical Centre, in Amsterdam, The Netherlands; Anita CE Vreugdenhil, with Department of Paediatrics, University Hospital Maastricht in Maastricht, The Netherlands; and Ilma Korponay-Szabo, with the Department of Paediatrics, University of Debrecen in Hungary, and the Paediatric Research Centre, University of Tampere, in Tampere, Finland.
Source:
- Open Original Shared Link




Recommended Comments