
Celiac.com 08/13/2022 - Celiac disease is an autoimmune gastrointestinal disorder that may occur in genetically susceptible individuals triggered by the ingestion of gluten-containing grains such as wheat, barley and rye. Of the many autoimmune disorders, celiac disease represents one of the few where the etiological agent is known and the disease subsides and goes in remission once the etiological agent is withdrawn from the diet.
Celiac disease is characterized by malabsorption resulting from inflammatory injury to the small intestinal mucosa, which, when prolonged, can cause malnutrition. The classic symptoms of celiac disease include diarrhea, weight loss and malnutrition. However, only a small percentage of patients with celiac disease present with the classic symptoms. Consequently, the clinical spectrum of celiac disease has grown much broader to include patients without classic symptoms. It is not uncommon for the initial symptoms to be non-gastrointestinal or for gastrointestinal symptoms, if present, to be mild or intermittent. Some of the common non-gastrointestinal manifestations include short stature, iron and folate deficiency, anemia, bone loss, aphthous stomatitis, arthralgia, dental enamel defects, etc. The inclusion of a wider range of clinical presentation has led to greater numbers of individuals diagnosed with celiac disease later in life than ever before. Adults may present with iron deficiency, macrocytic anemia and hypocalcaemia.
Celiac.com Sponsor (A12):
Studies have found the prevalence of celiac disease to be highly variable from population to population (1) and the true prevalence has been difficult to ascertain. The disparate criteria used in the diagnosis of celiac disease are often the cause. If only the clinical criteria are used in determining prevalence, the incidence of celiac disease is much lower as compared with incidence established by serological methods (2) . Using serological methods of diagnosis, the incidence of celiac disease in the general population is approximately one in 200.
Diagnosis of celiac disease based on clinical criteria can therefore be misleading and may lead to serious delays in proper diagnosis. Frequently, delays in diagnosis extend 10-13 years from the first clinical presentation of symptoms. Failure to diagnose celiac disease early on may predispose an individual to long-term complications such as splenic atrophy and intestinal lymphoma. The incidence of lymphoma arising in the context of celiac disease is difficult to ascertain. One study has shown incidence of lymphoma involving the gastrointestinal tract in patients with celiac disease to range from 3.6 percent to 40 percent (3) . In another recent study, celiac disease was found to be associated with significantly elevated risk for intestinal lymphoma, especially for non-Hodgkin’s (4) . A gluten-free diet normalizes the mucosa and helps reduce the malignant potential.
Histological examination of the small intestinal biopsy is considered to be the gold standard for diagnosing celiac disease, but it has its own limitations. Certain studies have shown some patients with latent or even active celiac disease that may have normal histopathology(5).
Serological Methods of Detecting Celiac Disease
The revised European Society of Pediatric Gastroenterology and Nutrition (ESPGAN) criteria for diagnosis of celiac disease include only a single biopsy with clear-cut remission of clinical symptoms on a gluten-free diet (6). Positive serology at the time of diagnosis with a decline in antibody levels on a gluten-free diet contributes to the diagnosis. The various serological tests employed in the work-up of patients suspected to have celiac disease include anti-gliadin antibody (AGA), anti-endomysial antibody (EMA), anti-reticulin antibody (ARA) and anti-tissue transglutaminase (tTG) antibody tests. Antibodies to gliadin and tTG are detected by the ELISA method, whereas endomysial and reticulin antibodies are detected by indirect immunofluorescence. EMA are very specific indicators of celiac disease. One study (7) concludes that “EmA-IgA is 100 percent sensitive and specific in active, untreated IgA-sufficient celiac disease patients when performed by an established laboratory.”
tTG has been identified as the endomysial antigen. This discovery has enabled development of automatable ELISA methods for detecting antibodies in the sera of patients with celiac disease. Many laboratories have opted to use the tTG antibody method in screening for celiac disease. In these laboratories, it may be the only assay used for detection of celiac disease cases. Various studies on the efficacy of the tTG antibody method for screening have found the specificity and sensitivity of this method to range from 90 percent to 95 percent (8). Assays using human tTG have been described to improve the sensitivity of detection of tTG antibodies. Surprisingly, in a recent report by the Medicines and Healthcare Products Regulatory Agency (MHRAM) on various anti-tTG IgA isotype assays the specificities were found to be good but assay sensitivity was often poor, indicating considerable variation in reliability of detection.
One limitation of existing serological methods is that, with the exception of IgG–gliadin, they detect only the IgA isotype of the antibodies; hence, IgA deficient celiac disease patients may yield false negative serology (9) . This may compromise the utility of the serum antibody methods in detecting all celiac disease cases (10).
What is IgA deficiency?
In the blood there are proteins called immunoglobulins that generally provide immunological protection. There are five types of immunoglobulins, known as IgG, IgA, IgM, IgE, and IgD. IgG and IgM provide protection in the circulatory system whereas IgA is transported to the surface of mucosal surfaces such as in the gastrointestinal tract and oral mucosa, safeguarding these mucosal surfaces from infection. Certain individuals that fail to produce the IgA immunoglobulin are referred to have selective IgA deficiency. The cause of this selective IgA deficiency is not known. What is known is that patients with selective IgA deficiency have a defect in differentiating B cells (one of the white cell types) into cells that manufacture immunoglobulins called plasma cells. The concentration of IgA in the plasma of normal individuals is about 300 mg/dl. In individuals with selective IgA deficiency the IgA levels are less than 0.05 mg/dl. IgA deficiency is one of the most common immunodeficiencies, found in one in 500-700 healthy blood donors (11). In most situations, these IgA deficient individuals are healthy. Those who develop symptoms suffer from sino-pulmonary infections, allergies, and autoimmune disorders, especially celiac disease (12). The incidence of IgA deficiency in celiac disease patients is between 2-3% representing a 10-15 fold increase over the general population. Familial inheritance of IgA deficiency occurs in 20% of cases. While the selective defect of B cells limits the number of IgA secreting plasma cells, IgA deficient individuals have normal function of IgG and other immunoglobulin secreting plasma cells. Celiac disease patients with IgA deficiency produce IgG immunoglobulin normally, and the antibodies to EMA, tTG and gliadin are of the IgG isotype rather than IgA. To prevent false negative results in IgA deficient cases of celiac disease, it is necessary to include serological methods that can detect antibodies of IgG isotype.
How can we detect Celiac Disease in patients with IgA deficiency?
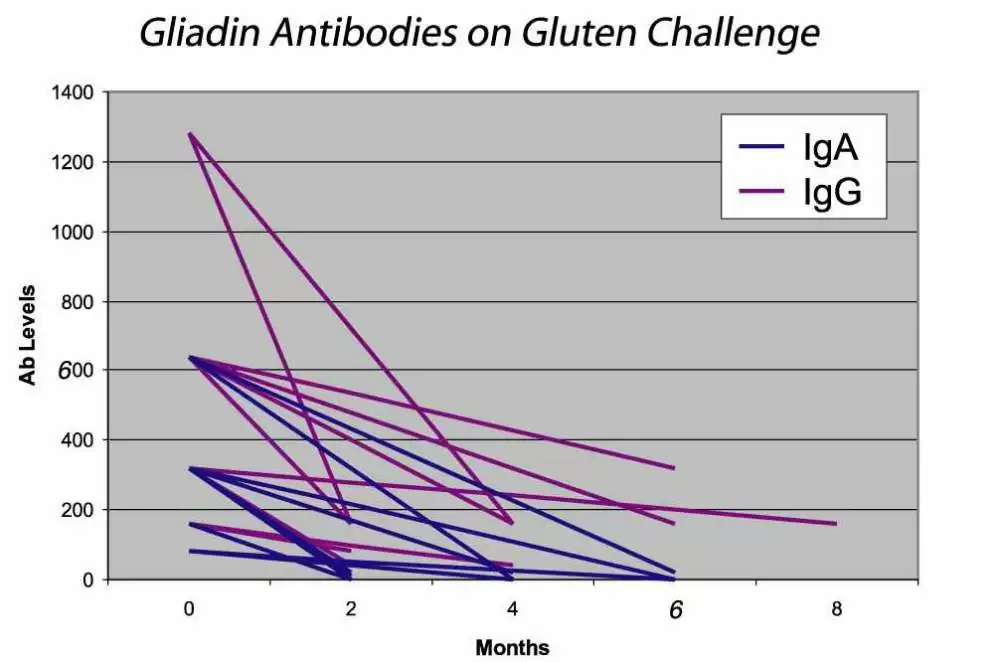
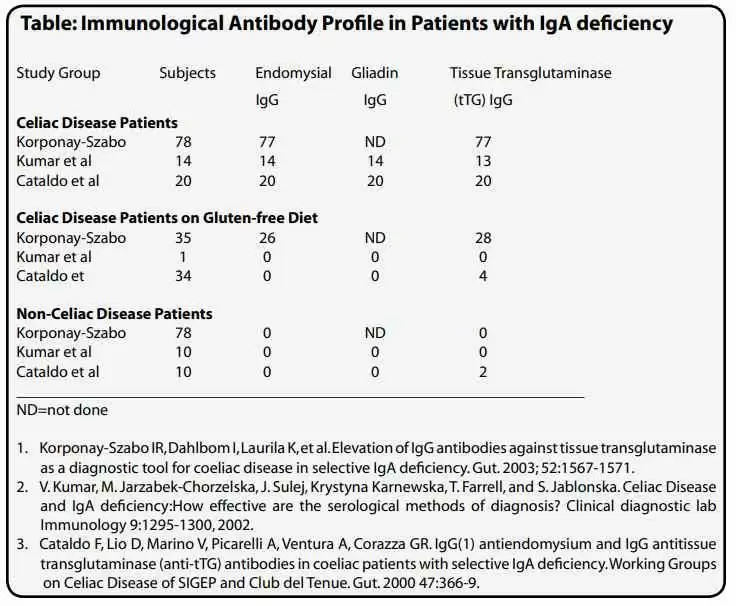
In patients with known selective IgA deficiency, the IgG antibody levels for EMA, tTG and gliadin can be measured and are very effective in identifying patients with celiac disease. However, one generally does not know if the individual is IgA deficient. Until specific tests for IgA levels are performed, the IgA status of an individual may never be known. As IgA deficiency is more prevalent in the celiac population than the general population, it has been proposed that all patients who are considered for celiac disease be tested for IgA levels to identify cases of IgA deficiency. Checking all routine samples referred to a laboratory for celiac disease testing, Lock and Unsworth found that testing for IgA levels in identifying IgA deficient celiac disease patients is excessive and more likely to identify non-celiac disease cases (13). They concluded that testing of IgA levels is not cost effective. Testing for IgG antibodies to EMA, tTG or AGA, however, is cost effective. Detection of these antibodies either individually or in combination helps to identify all cases of IgA deficient celiac disease on normal diets. Using this method, we reported the first IgA deficient celiac disease case in 1989. Since then, others have reported on large populations of IgA deficient celiac disease and non-celiac disease cases and found the serological methods to be effective (see table on pg. 17). The levels of these antibodies are also of interest, as antibody level tends to correlate with the severity of the disease. When a patient is on a gluten free diet, the levels of these antibodies will decrease and eventually disappear, suggesting that the patient is in remission. Thereafter, tests for antibody levels could be checked annually or bi-annually to ensure the individual’s dietary compliance. Intake of gluten in individuals who are in remission will result in the re-appearance or increase of these antibodies in the serum (see figure below).






Recommended Comments
There are no comments to display.
Create an account or sign in to comment
You need to be a member in order to leave a comment
Create an account
Sign up for a new account in our community. It's easy!
Register a new accountSign in
Already have an account? Sign in here.
Sign In Now