.webp.09959191de09d61c3bda827676150d09.webp)
Celiac.com 05/06/2013 - After a diagnosis of celiac disease, it is unlikely a gluten-free diet alone will provide expeditious relief from its various associated symptoms and health problems. Additional steps and remedies to restore the integrity of the damaged intestinal mucosal barrier are often needed, at least, to hasten the process. The first step all patients should take after a celiac disease diagnosis is to establish a "baseline" measurement of the intestinal damage which can be used to assess how well the gut is healing over time. Assessing the status of the gut via multiple endoscopic biopsy procedures is not a very practical choice of method. It is expensive, invasive, uncomfortable, subject to possible risks and complications, and only assesses the intestinal mucosa at the sites biopsied.[1] The downsides of biopsies can be avoided by using a low-cost, non-invasive leaky gut or intestinal permeability test to provide a useful baseline measurement and following up with future periodical testing for comparison.
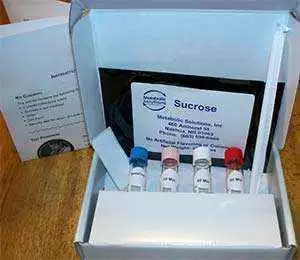 A new, simple and easy-to-use home Carbon-13 or 13C-Sucrose Breath Test to assess for leaky gut is now available. The 13C-Sucrose Breath Test takes only 90 minutes, requiring drinking a sucrose (20 grams) solution and collecting 4 breath samples 30 minutes apart following an 8 hour fast. 13C-Sucrose Breath Test samples, collected in 4 small screw-capped glass tubes, are simply mailed to the laboratory within 14 days in the original shipping box that also serves as a pre-paid U.S. Postal Service First Class Mailer. 13C-Sucrose Breath Test results are emailed back within 24 hours after the breath test samples are received.[2]
A new, simple and easy-to-use home Carbon-13 or 13C-Sucrose Breath Test to assess for leaky gut is now available. The 13C-Sucrose Breath Test takes only 90 minutes, requiring drinking a sucrose (20 grams) solution and collecting 4 breath samples 30 minutes apart following an 8 hour fast. 13C-Sucrose Breath Test samples, collected in 4 small screw-capped glass tubes, are simply mailed to the laboratory within 14 days in the original shipping box that also serves as a pre-paid U.S. Postal Service First Class Mailer. 13C-Sucrose Breath Test results are emailed back within 24 hours after the breath test samples are received.[2]
Celiac.com Sponsor (A12):
In comparison, the better known and established Lactulose/Mannitol Intestinal Permeability Test requires an 8 hour overnight fast, collecting a pretest urine specimen, drinking a Lactulose(5 grams)/Mannitol(1 gram) solution, drinking water every 2 hours, and sampling and collecting all the urine excreted over a 6 hour period. The Lactulose/Mannitol Test urine specimens must be kept refrigerated and shipped to and received by the laboratory within 24 hours in a special frozen gel pack shipper via FedEx overnight delivery. Lactulose/Mannitol Test results are emailed around 7 days after the specimens are received.[3]
The 13C-Sucrose Breath Test is based on the level of sucrase activity in cells of the intestinal mucosa. Sucrase enzymes break down sucrose into its constituent components, fructose and glucose, which, when metabolized in the liver, produce carbon dioxide (CO2) exhaled in the breath. Sucrase enzymes are synthesized and embedded in the "brush border" of cells comprising the villi of the intestinal mucosa. The brush border is composed of numerous microvilli which extend into the intestinal lumen from the face of the villus cells.[1,4,5]
Natural sucrose from cane sugar contains 2 forms of carbon atoms, carbon-12 (12C) and the stable, non-radioactive isotope, carbon-13 (13C). 12C and 13C carbon dioxide can be detected and measured using an isotope ratio mass spectrometer (IRMS). By using natural 13C-enriched sucrose in which the ratio of 13C to 12C has been previously measured, measurement and analysis of the relative amounts of 13C and 12C in the carbon dioxide exhaled by an individual after ingesting a known quantity of the 13C-enriched sucrose provides an indicator of the sucrase activity in the brush border. Damage to villus cells and the brush border results in both a decrease in sucrase activity and an increase intestinal permeability. This forms the basis for a leaky gut test where low carbon-13 measured in the breath means the intestinal villi are damaged.[1,4]
The Metabolic Solutions, Inc. 13C-Sucrose Breath Test is taken after a minimum 8 hour fast. No sleep or exercise is allowed during the test. A baseline breath sample is first collected. Then a 20 gram packet of 13C-enriched sucrose is stirred into an 8 ounce glass of water and ingested. Breath samples are then collected at 30, 60, and 90 minutes after sucrose ingestion. Breath samples are collected into 4 small labeled 10 ml glass tubes with color-coded screw caps by simply unscrewing the cap, taking a full breath, and blowing into the tube through an ordinary straw. Breath will be felt escaping out of the tube as it is blown through the straw. That is normal. All that is required is to screw the cap back on the tube, finger-tight, within 5 seconds. If screwing the cap back on takes more than 5 seconds, one can simply take another breath and blow again. After the test, the 4 tubes and the instruction sheet, filled-in with the test taker's name, email address, date of birth, sex, height and weight, are repacked into the original shipping box and dropped into any U.S. mail box within 14 days. A prepaid U.S. Postal Service First Class mailing label is printed on the box. Test results are emailed back within 24 hours after receipt by Metabolic Solutions, Inc. If the percentage cumulative dose of 13C recovered from the sucrose at 90 minutes is less than 5.10% 13C for females or less than 3.91% 13C for males, the test is considered positive for intestinal damage.[2,4]
The percentage cumulative dose of 13C recovered from sucrose over 90 minutes is calculated from the area under the curve formed by plotting the amount 13C carbon dioxide measured in each 10 ml breath sample against time, the ratio of 13C to 12C in the 20 grams of sucrose ingested, and a formula where age, sex, height and weight are used in a to compute the total amount of carbon dioxide (both 12C and 13C) that would be expirated by the test subject during the test time period. Together, these considerations yield the desired test result expressing how much of the 20 grams of sucrose ingested has been metabolized by brush border sucrase enzymes and the liver and expelled in the breath within 90 minutes.[6,7]
The 13C-Sucrose Breath Test was invented and developed by scientists at The Centre for Paediatric and Adolescent Gastroenterology, Children, Youth and Women’s Health Service, University of Adelaide, North Adelaide, SA Australia. U.S. Patent 7338454 (March 4, 2008) and U.S. Patent Application 20080160504 (July 3, 2008) are assigned to Children, Youth and Women's Health Service Incorporated, North Adelaide, AU.[7,8] Development of the 13C-Sucrose Breath Test began prior to 2002, the year an international patent application for the test was filed.[9] Only recently has the test become available in the United States. Because of limited published clinical studies, the 13C-Sucrose Breath Test has not yet become fully established as a standard for intestinal permeability testing, but has advantages over other tests and appears to be reliable. Except in cases of intestinal damage, sucrase levels generally remain consistent thoughout life and across differing ethnicities. Only 0.2% of the population has a genetic sucrase deficiency.[1,4,7]
In only one study concerning zinc absorption in children with celiac disease has the 13C-Sucrose Breath Test been compared against Marsh scores of celiac disease patients. That study recruited 51 children, aged 2 to 18 years, who underwent routine endoscopy for the diagnosis or exclusion of celiac disease. There were no significant differences in 13C-Sucrose Breath Test results between children with a Marsh score of 0 and Marsh scores of 3a, 3b, and 3c. All participants, including those with Marsh score 0, had a mean percentage cumulative dose of 13C recovered from sucrose at 90 minutes slightly below the 5.06% cut-off for a normal 13C-Sucrose Breath Test. In a prior study, the average test result for normal healthy children (aged 11.2 +/- 0.8 years) was 8.5%. Since all recruited children had bowel symptoms prompting an endoscopy, those children with March scores of 0, while not diagnosed with celiac disease, were not normal and healthy. The study shows the 13C-Sucrose Breath Test is potentially useful to monitor gut integrity in celiac disease children.[10]
Lactulose/Mannitol and the similar Lactulose/Rhamnose dual-sugar permeability tests have been studied since 1979. Mannitol and rhamnose are small sugar molecules which normally cross the intestinal barrier via absorption through healthy intestinal cell membranes. Lactulose is a large sugar molecule that crosses the intestinal barrier by passing between intestinal cells through the "tight junctions". These sugars are non-metabolizable, and, therefore, the molecules which cross the intestinal barrier are excreted unchanged in the urine. Loss of tight junctions elevates lactulose urinary levels. Damage to intestinal cell membranes compromises absorption of mannitol or rhamnose molecules, and, thus, lowers mannitol or rhamnose urinary levels. A high urinary lactulose to mannitol or lactulose to rhamnose ratio, therefore, indicates intestinal damage.[1]
Lactulose/Mannitol permeability tests have been studied in celiac disease patients with mixed results as to whether the test is suitable as a screening test for celiac disease.[11-15] Like the 13C-Sucrose Breath Test, the Lactulose/Mannitol test is probably best used to monitor the progress of gut healing by first testing intestinal permeability at the time of celiac disease diagnosis to establish a baseline for subsequent follow-up tests.
The two tests are available online at competitive prices. Metabolic Solutions Inc. currently offers the 13C Sucrose Breath Test for $129 which includes shipping. The Genova Diagnostics Lactulose/Mannitol Intestinal Permeability Test is available at similar discounted prices from several online sellers.
13C-Sucrose Breath Leaky Gut Test Kits are available directly from:
Metabolic Solutions, Inc.
460 Amherst Street
Nashua, NH 03063
866-302-1998
For Health Professionals:
Open Original Shared Link
To Order Home Test Kits:
Open Original Shared Link
Lactulose/Mannitol Intestinal Permeability Test Kits are available (through retail website sellers) from:
GDX/Genova Diagnostics
63 Zillicoa Street
Asheville, NC 28801
800-522-4762
Open Original Shared Link
I have personally taken the Metabolic Solutions, Inc. 13C-Sucrose Breath Leaky Gut Test. A kit ordered online on a Monday was picked up from my post office box on Friday of the same week. The test was taken on the following Monday, and the prepaid shipping box was dropped off at the U.S. Post Office the next day, Tuesday. The test result was received in an email sent from Metabolic Solutions, Inc. Thursday evening, only 2 days later. The test result was normal, 6.97% cumulative dose of 13C recovered, well above the cut-off of 3.91% for males.
Since no such simple and easy leaky gut test existed more than a dozen years ago when leaky gut was first suspected, no baseline was ever established for test result comparison. Following years of diet and supplements targeted to heal leaky gut and treat its symptoms, a general indication of current gut status was desired. Some symptoms still remain. A normal test result suggests the symptoms are likely due to other factors occurring before or during the leaky gut healing process, such as inflammation due to the continued presence of previously "leaked" environmental toxins accumulated in adipose tissue.
Sources
1. Mucositis and non-invasive markers of small intestinal function.
Tooley KL, Howarth GS, Butler RN.
Cancer Biol Ther. 2009 May;8(9):753-8.
Open Original Shared Link
2. Instructions for Leaky Gut Breath Test.
Metabolic Solutions, Inc.
Open Original Shared Link
3. GDX Lactulose/Mannitol Intestinal Permeability Test Kit Instructions.
Genova Diagnostics.
Open Original Shared Link
4. 13C-Sucrose Breath Test for Leaky Gut Syndrome.
Metabolic Solutions, Inc.
Open Original Shared Link
5. Small Intestine: Brush Border Enzymes.
R. Bowen.
Pathophysiology of the Digestive System, Colorado State University.
Open Original Shared Link
6. Recent Results of the Development and Application of 13C–Breath Tests.
Klaus Wetzel and Heinz Fischer.
Fischer ANalysen Instrumente GmbH (FAN), Leipzig, December 1999, Page 33.
Open Original Shared Link
7. Breath Test.
Butler RN, Tivey D, Davidson GP, Pelton N.
U.S. Patent 7338454, March 4, 2008.
Open Original Shared Link
8. Breath Test.
Butler RN, Tivey D, Davidson GP, Pelton N.
U.S. Patent Application 20080160504, July 3, 2008.
Open Original Shared Link
9. Breath test.
Butler RN, Tivey D, Davidson GP, Pelton N.
International Application No.: PCT/AU2002/001666, December 9, 2002.
10. Zinc homeostasis and gut function in children with celiac disease.
Tran celiac disease, Katsikeros R, Manton N, Krebs NF, Hambidge KM, Butler RN, Davidson GP.
Am J Clin Nutr. 2011 Oct;94(4):1026-32.
Open Original Shared Link
11. Follow-up of adult celiac patients: which noninvasive test reflects mucosal status most reliably?
Vecsei AK, Graf UB, Vogelsang H.
Endoscopy. 2009 Feb;41(2):123-8.
Open Original Shared Link
12. Intestinal permeability in long-term follow-up of patients with celiac disease on a gluten-free diet.
Duerksen DR, Wilhelm-Boyles C, Parry DM.
Dig Dis Sci. 2005 Apr;50(4):785-90.
Open Original Shared Link
13. Lactulose-mannitol intestinal permeability test: a useful screening test for adult coeliac disease.
Johnston SD, Smye M, Watson RG, McMillan SA, Trimble ER, Love AH.
Ann Clin Biochem. 2000 Jul;37 ( Pt 4):512-9.
Open Original Shared Link
14. Lactulose-mannitol intestinal permeability test: a useful screening test for adult coeliac disease.
Johnston SD, Smye M, Watson RG, McMillan SA, Trimble ER, Love AH.
Ann Clin Biochem. 2000 Jul;37 ( Pt 4):512-9.
Open Original Shared Link
15. Is the sugar intestinal permeability test a reliable investigation for coeliac disease screening?
Catassi C, Fabiani E, Rätsch IM, Bonucci A, Dotti M, Coppa GV, Giorgi PL.
Gut. 1997 Feb;40(2):215-7.
Open Original Shared Link






Recommended Comments
There are no comments to display.